SporeVax® utilises bacterial spores as a vaccine delivery agent. Spores are dormant life forms produced by some bacteria. They are unique in being heat stable and able to survive indefinitely at temperatures as high as 70°C. In our case, we use the organism known as Bacillus subtilis because of its current use as a food ingredient and the extensive research conducted with this organism over the last 40 years. Indeed, B. subtilis is probably one of the best understood of all bacteria. SporeGen®’s approach has been to exploit Bacillus as the delivery agent to enhance the body’s natural immune responses. Vaccine antigens can be displayed on the surface of Bacillus. The modified spores are inactivated then used for immunisation.
In the USA, B. subtilis has been given self-affirmed GRAS (Generally Regarded As Safe) status and in Europe the European Food Safety Authority (EFSA) has designated B. subtilis as QPS, giving it a Qualified Presumption of Safety. Many indigenous foods throughout the world have traditionally contained B. subtilis and it is used in many probiotic-type products available today.
Alternatively the Bacillus spore is first killed and adsorbed with antigen and then used for immunisation.
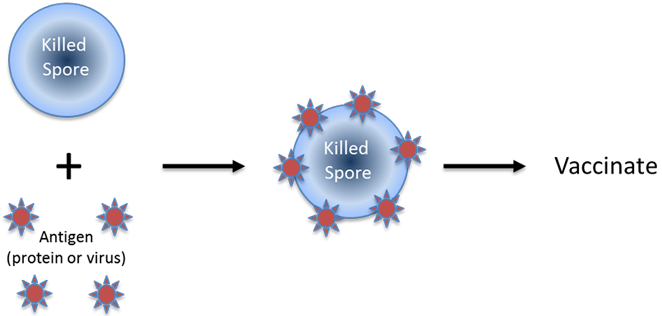
In both approaches, the vaccine is delivered by one of three routes: oral, sub-lingual or nasal, depending upon the individual vaccine requirements. The SporeVax® approach has the following superior attributes compared with other vaccine delivery systems under development:
- Bacillus spores are heat stable, so the vaccine requires no cold chain for storage and transportation.
- Oral, sub-lingual and nasal delivery is needleless and convenient.
- Mucosal immunity is desirable for many human and animal diseases: these immune responses are strongest when the vaccine is delivered by a mucosal route (by mouth or by nose). Mucosal immunity is the most difficult to achieve due to the composition of many vaccines in use today: the use of spores is a major breakthrough.
- Bacillus spores carry a natural adjuvant property that enhances the body’s natural immune responses. Typically, they produce balanced Th1 and Th2 responses as well as stimulating the innate immune system, an important primer for long-term immunity.
- Compared with most other new vaccine systems under development, spores are substantially more simple and straightforward to produce.
- A strong patent portfolio.
- SporeGen® uses a unique isolate of B. subtilis isolated from humans. This strain is well characterised and owned by us.
Preclinical Studies using SporeVax®
| Delivery Route | Protection | |
|---|---|---|
| Tetanus | Oral | |
| Nasal | ||
| Clostridium perfringens | Oral | |
| Nasal | ||
| Anthrax | Nasal | |
| Influenza | Nasal | |
| Clostridium difficile | Oral | |
| Sub-lingual |
Song, M., H. A. Hong, J.-M. Huang, C. Colenutt, D. D. Khang, N. T. Van Anh, S.-M. Park, B.-S. Shim, H. H. Song, I. S. Cheon, J. E. Jang, J.-A. Choi, Y. K. Choi, K. Stadler & S. M. Cutting, (2012) Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine. 30: 3266-77.
Patima Permpoonpattana, Huynh A. Hong, Jutarop Phetcharaburanin, Jen-Min Huang, Jenny Cook, Neil F. Fairweather & Simon M. Cutting. (2011). Immunisation with Bacillus spores expressing toxin A peptide repeats protects against infection with toxin A+ B+ strains of Clostridium difficile. Infection and Immunity. 79: 2295-2302.
Huang, J. M., H. A. Hong, H. V. Tong, T. H. Hoang, A. Brisson, and S. M. Cutting. (2010). Mucosal Delivery of Antigens Using Adsorption to Bacterial Spores. Vaccine 28: 1021-1030.
Li Hua Yu & Simon M. Cutting. The Effect of Anti-Spore Antibody Responses on the Use of Spores for Vaccine Delivery. (2009) Vaccine. 34: 4576-4584.
Jen-Min Huang, Roberto M. La Ragione, William A. Cooley, Stephen Todryk & Simon M. Cutting (2008). Cytoplasmic Delivery of Antigens by Bacillus subtilis enhances Th1 Responses. Vaccine. 26: 6043-6052.
Hoang, T. H., Hong, H.A., Clarke, Graeme. C., Titball, R.W., Cutting, S.M. (2008) A Recombinant Bacillus subtilis Expressing the C. perfringens Alpha-Toxoid is a Candidate, Orally Delivered, Necrotic Enteritis Vaccine. Infection & Immunity. 76: 5257-65.
Huang JM, La Ragione RM, Nunez A, Cutting SM. 2008. Immunostimulatory activity of Bacillus spores. FEMS Immunol. Med. Microbiol. 53: 195-203.
Nguyen Q. Uyen, Huynh A. Hong and Simon M. Cutting. 2007. Enhanced Immunisation and Expression Strategies using Bacterial Spores as Heat-Stable Vaccine Delivery Vehicles. Vaccine. 25: 356-365.
Huynh A. Hong, Helen S. Atkins, Helen C. Flick-Smith, Le H. Duc, Zarmina Durrani, Sjoerd Rijpkema, Richard W. Titball and Simon M. Cutting. 2007. Immunization against anthrax using Bacillus subtilis spores expressing the anthrax protective antigen. Vaccine. 25: 346-355.
Tam, N.M.K., Uyen, N.Q., Hong, H.A., Duc, L.H., Hoa, T.T., Serra, C. H., Henriques, A.O. and Cutting, S.M. 2006. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriology. 188: 2692-2700.
Duc L. H., H. A. Hong, N. Q. Uyen, and S. M. Cutting. 2004. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine 22:1873-85.
Mauriello, E. M., H. Duc le, R. Isticato, G. Cangiano, H. A. Hong, M. De Felice, E. Ricca, and S. M. Cutting. 2004. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 22:1177-87.
Duc L. H., H. A. Hong, and S. M. Cutting. 2003. Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen delivery. Vaccine 21:4215-24.
Duc L. H., H. A. Hong, N. Fairweather, E. Ricca, and S. M. Cutting. 2003. Bacterial spores as vaccine vehicles. Infect Immun 71:2810-8.
R. Relic, L. Sibley, JM Huang, I. Pepponi, A. Hoppe, HA Hong and S.M. Cutting. (2013) Mucosal Vaccination against TB Using Inert Bioparticles. Infection and Immunity. 81: 4071-80.
